Introducing the Abundance of Isotopes Chem Worksheet 4-3, an enthralling journey into the fascinating realm of isotopes and their profound implications in chemistry. This worksheet serves as a comprehensive guide, unraveling the intricacies of isotopes, their types, abundance, and diverse applications.
From the fundamental concepts of isotopes to their groundbreaking applications in medicine, industry, and research, this worksheet delves deep into the world of isotopes, empowering learners with a comprehensive understanding of these remarkable elements.
Abundance of Isotopes: Abundance Of Isotopes Chem Worksheet 4-3
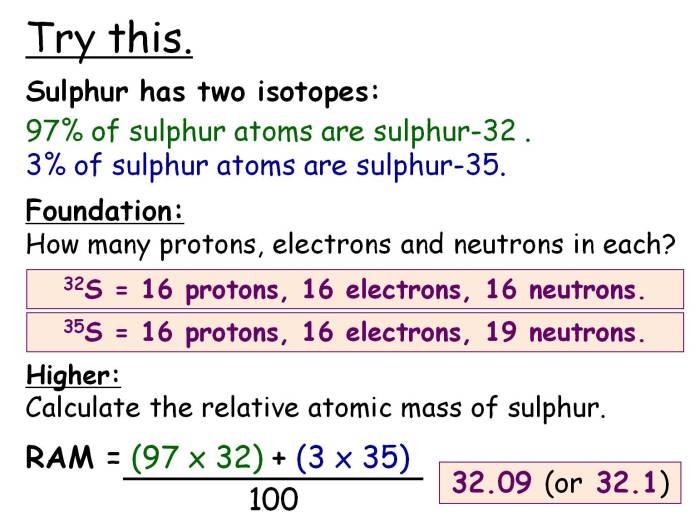
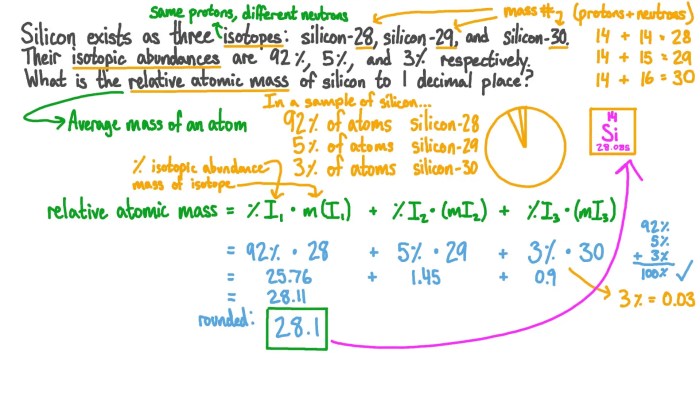
The abundance of isotopes in nature is determined by several factors, including the stability of the isotopes, the rate of their production, and the rate of their decay. Stable isotopes are those that do not undergo radioactive decay, while radioactive isotopes are those that do.
The rate of production of an isotope is determined by the nuclear reactions that produce it, and the rate of its decay is determined by its half-life.
The abundance of isotopes in the environment can be measured using a variety of techniques, including mass spectrometry and atomic absorption spectroscopy. Mass spectrometry is a technique that separates isotopes based on their mass-to-charge ratio, while atomic absorption spectroscopy is a technique that measures the amount of light absorbed by an element at specific wavelengths.
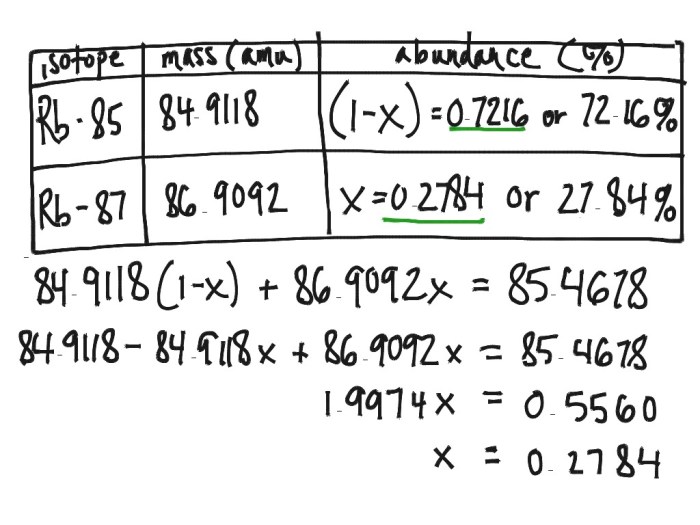
Isotopic Ratios
Isotopic ratios are the ratios of the abundances of different isotopes of the same element. Isotopic ratios can be used to determine the age of materials, the source of materials, and the processes that have occurred in the environment. For example, the ratio of carbon-14 to carbon-12 in a sample can be used to determine the age of the sample, while the ratio of strontium-87 to strontium-86 in a sample can be used to determine the source of the sample.
Examples of Isotope Abundance, Abundance of isotopes chem worksheet 4-3
- Hydrogen has three isotopes: protium (¹H), deuterium (²H), and tritium (³H). Protium is the most abundant isotope, accounting for 99.985% of all hydrogen atoms. Deuterium is the second most abundant isotope, accounting for 0.015% of all hydrogen atoms. Tritium is the least abundant isotope, accounting for only 1 atom in 10¹⁸ hydrogen atoms.
- Carbon has three naturally occurring isotopes: carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C). Carbon-12 is the most abundant isotope, accounting for 98.89% of all carbon atoms. Carbon-13 is the second most abundant isotope, accounting for 1.11% of all carbon atoms.
Carbon-14 is the least abundant isotope, accounting for only 1 atom in 10¹² carbon atoms.
- Oxygen has three stable isotopes: oxygen-16 (¹⁶O), oxygen-17 (¹⁷O), and oxygen-18 (¹⁸O). Oxygen-16 is the most abundant isotope, accounting for 99.757% of all oxygen atoms. Oxygen-17 is the second most abundant isotope, accounting for 0.038% of all oxygen atoms. Oxygen-18 is the least abundant isotope, accounting for 0.205% of all oxygen atoms.
Essential Questionnaire
What are isotopes?
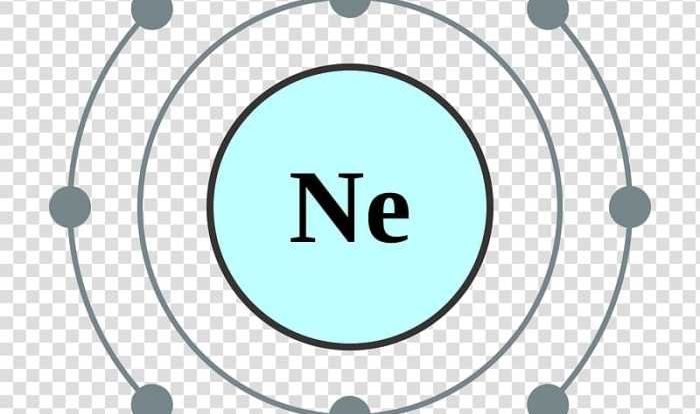
Isotopes are variations of the same element that share the same atomic number but differ in their neutron count, resulting in different atomic masses.
How are isotopes used in medicine?
Isotopes such as iodine-131 are used in medical imaging and treatment, while cobalt-60 is employed in radiation therapy.
What is the significance of isotopic ratios?
Isotopic ratios provide valuable insights into geological processes, climate change, and the origin of life on Earth.