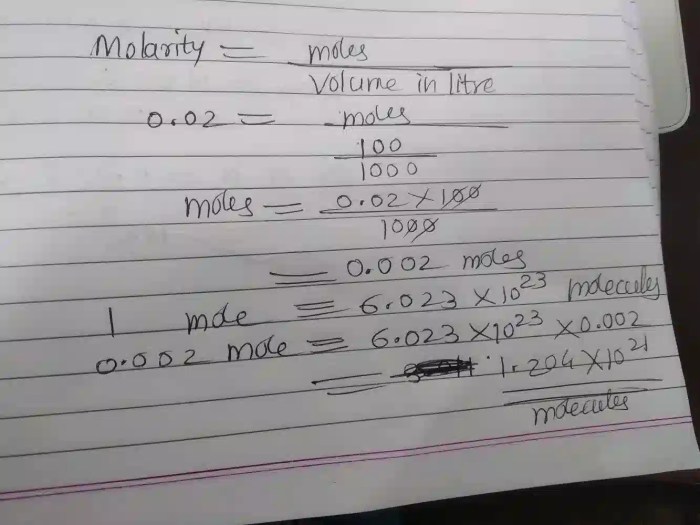How many molecules are in 24 grams of fef3 – Embarking on a scientific inquiry, we delve into the intriguing question: how many molecules reside within 24 grams of FeF3? This exploration unveils the fundamental concepts of molecular composition, molar mass, and the conversion between mass and moles, ultimately guiding us towards a precise determination of the number of molecules present in this intriguing compound.
How Many Molecules Are in 24 Grams of FeF3?

Molecular Composition of FeF3
FeF3 is an inorganic compound with the chemical formula iron(III) fluoride. It is a salt composed of iron and fluorine ions. The molecular structure of FeF3 consists of a central iron(III) ion (Fe3+) surrounded by three fluoride ions (F-) in a trigonal pyramidal arrangement.
Molar Mass Calculation
Molar mass is the mass of one mole of a substance. It is calculated by adding the atomic masses of all the atoms in the molecular formula. The atomic mass of iron is 55.845 g/mol, and the atomic mass of fluorine is 18.998 g/mol.
Therefore, the molar mass of FeF3 is:Molar mass of FeF3 = (1 x 55.845 g/mol) + (3 x 18.998 g/mol) = 159.69 g/mol
Converting Mass to Moles
Avogadro’s number is the number of atoms or molecules in one mole of a substance, which is 6.022 x 10^
To convert mass to moles, we divide the mass by the molar mass:
Moles of FeF3 = Mass / Molar massMoles of FeF3 = 24 g / 159.69 g/mol = 0.15 moles
Determining the Number of Molecules, How many molecules are in 24 grams of fef3
The number of molecules in a given amount of substance can be calculated by multiplying the number of moles by Avogadro’s number:Number of molecules = Moles x Avogadro’s numberNumber of molecules = 0.15 moles x 6.022 x 10^23 molecules/mol = 9.033 x 10^22 molecules
Common Queries
What is the chemical formula of FeF3?
FeF3 represents the chemical formula of iron(III) fluoride, an inorganic compound composed of iron and fluorine atoms.
How do I calculate the molar mass of FeF3?
To calculate the molar mass, add the atomic weights of each element multiplied by their respective number of atoms in the chemical formula. For FeF3, the molar mass is approximately 159.69 g/mol.
How many moles are present in 24 grams of FeF3?
Using the molar mass, we can determine that 24 grams of FeF3 is equivalent to approximately 0.15 moles.