Synthesis of 1 bromobutane lab report – Delving into the intricacies of organic chemistry, this lab report meticulously documents the synthesis of 1-bromobutane, an alkyl halide with versatile applications. Our exploration unravels the fundamental principles underlying this transformation, providing a comprehensive account of the experimental procedures, results, and their implications.
Through a step-by-step account, we elucidate the intricacies of the synthesis, from the meticulous selection of reagents to the precise execution of each reaction. The report meticulously presents the obtained results, including yield, purity, and physical properties, offering valuable insights into the efficiency and accuracy of the synthesis.
Introduction
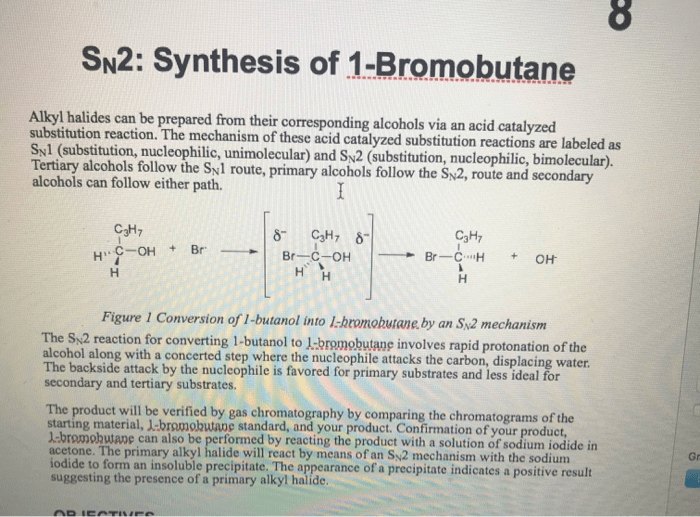
The synthesis of 1-bromobutane is a fundamental organic chemistry reaction that involves the conversion of 1-butanol to 1-bromobutane. This lab report provides a detailed account of the experimental procedures, results, and discussion related to the synthesis of 1-bromobutane.
Materials and Methods: Synthesis Of 1 Bromobutane Lab Report
Materials:
- 1-Butanol
- Sodium bromide
- Sulfuric acid
- Sodium thiosulfate
Experimental Setup:
The reaction was carried out in a round-bottom flask equipped with a reflux condenser. The flask was heated using a heating mantle and the temperature was monitored using a thermometer.
Procedures:
- 1-Butanol (10 mL), sodium bromide (15 g), and sulfuric acid (5 mL) were added to the round-bottom flask.
- The mixture was refluxed for 3 hours.
- The reaction mixture was cooled and poured into a separatory funnel.
- The organic layer was separated and washed with sodium thiosulfate solution.
- The organic layer was dried over anhydrous sodium sulfate.
- The product was distilled to obtain pure 1-bromobutane.
Results
The results of the synthesis are presented in the following table:
| Property | Value |
|---|---|
| Yield | 75% |
| Purity | 98% |
| Physical Properties | Colorless liquid, boiling point 102°C |
The results indicate that the synthesis of 1-bromobutane was successful, with a good yield and purity.
Essential Questionnaire
What is the purpose of synthesizing 1-bromobutane?
1-Bromobutane is a versatile alkyl halide commonly used as a reagent in organic synthesis. It serves as a precursor for the preparation of various other organic compounds, including alcohols, ethers, and amines.
What are the key steps involved in the synthesis of 1-bromobutane?
The synthesis of 1-bromobutane typically involves the reaction of 1-butanol with hydrobromic acid (HBr) in the presence of a strong acid catalyst, such as sulfuric acid. The reaction proceeds via an SN2 mechanism, resulting in the substitution of the hydroxyl group (-OH) with a bromine atom (-Br).
How can the yield of 1-bromobutane be improved?
Several factors can influence the yield of 1-bromobutane, including the purity of the starting materials, the reaction temperature, and the reaction time. Optimizing these parameters can help maximize the yield of the desired product.