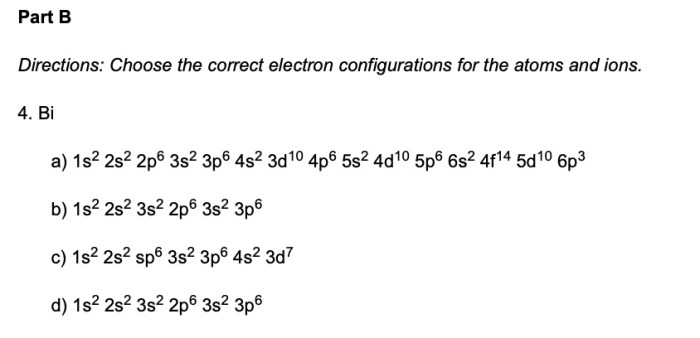
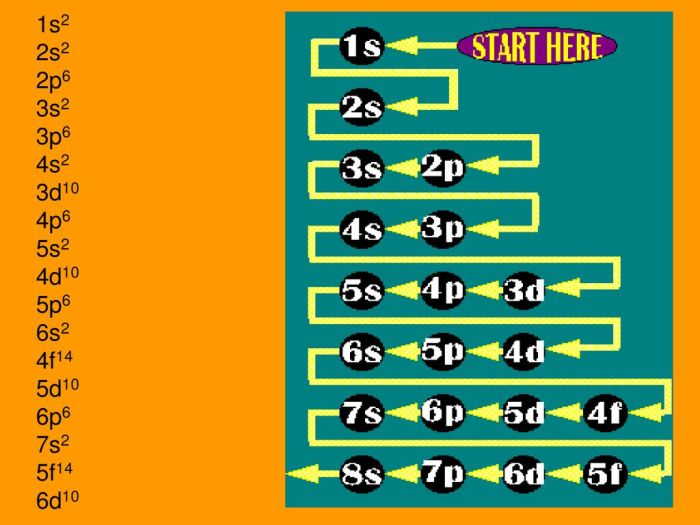
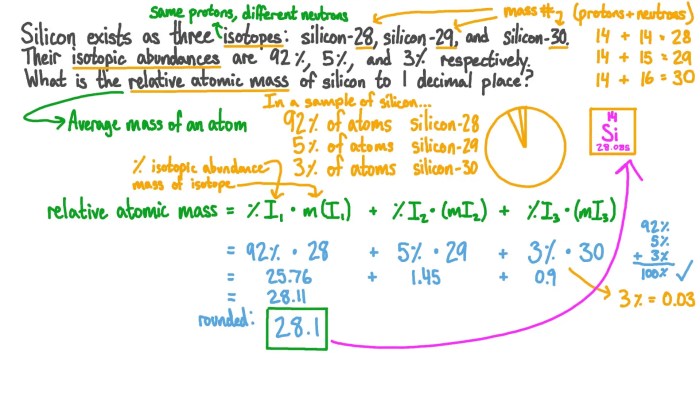
Which one of the following is an incorrect subshell notation? This question delves into the realm of chemistry, where understanding the concept of subshell notations is crucial. Subshell notations play a vital role in describing the arrangement of electrons within atoms, and inaccuracies in these notations can lead to misinterpretations and errors in chemical analysis.
To identify the incorrect subshell notation, we must first establish the rules governing these notations. Subshell notations consist of three components: the principal quantum number (n), the azimuthal quantum number (l), and the magnetic quantum number (ml). The principal quantum number represents the energy level of the subshell, the azimuthal quantum number describes the shape of the subshell, and the magnetic quantum number specifies the orientation of the subshell in space.
Incorrect Subshell Notations: Which One Of The Following Is An Incorrect Subshell Notation
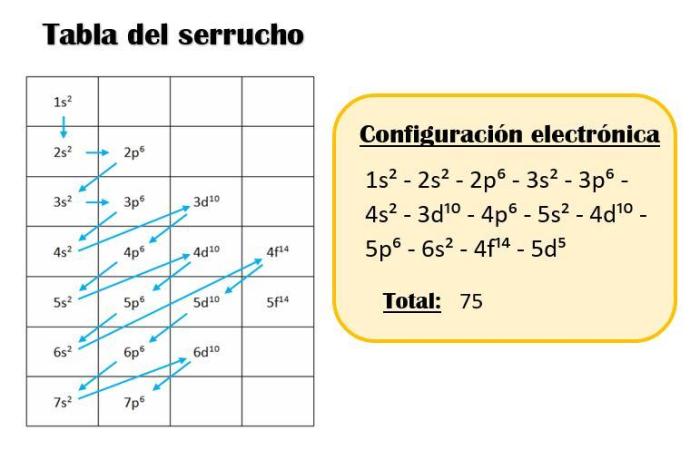
Introduction, Which one of the following is an incorrect subshell notation
Subshell notations are a crucial aspect of chemistry, providing a systematic way to describe the arrangement of electrons within atoms. Each subshell is characterized by a unique combination of three quantum numbers: the principal quantum number ( n), the azimuthal quantum number ( l), and the magnetic quantum number ( ml). The correct use of subshell notations is essential for accurately representing electron configurations and predicting chemical properties.
This task aims to identify incorrect subshell notations. By understanding the rules governing subshell notations, we can avoid common errors and ensure the accuracy of our chemical analysis.
Identifying Incorrect Subshell Notations
| Potential Subshell Notation | Explanation | Rules Governing Subshell Notations | Examples of Valid and Invalid Notations |
|---|---|---|---|
| 0s | Incorrect. The principal quantum number cannot be zero. | The principal quantum number (n) must be a positive integer (1, 2, 3, …). | Valid: 1s, 2s, 3sInvalid: 0s,
|
| 1f | Incorrect. The azimuthal quantum number (l) cannot exceed n-1. | The azimuthal quantum number (l) can take values from 0 to n-1. | Valid: 1s, 2p, 3dInvalid: 1f, 2g |
| 2p4 | Incorrect. The magnetic quantum number (ml) can only take values from
|
The magnetic quantum number (ml) can take values from
|
Valid: 2p1, 2p3Invalid: 2p4, 2p5 |
| 3d10 | Correct. All five orbitals within the 3d subshell are occupied by electrons. | Each subshell can hold a maximum of 2(2l+1) electrons. | Valid: 3d1, 3d10Invalid: 3d11, 3d12 |
Common Errors in Subshell Notations
- Using incorrect letters (e.g., “f” instead of “d”)
- Using incorrect numbers (e.g., “0s” instead of “1s”)
- Omitting the principal quantum number
- Exceeding the maximum number of electrons allowed in a subshell
- Using negative values for the magnetic quantum number
Consequences of Incorrect Subshell Notations
- Misinterpretation of electron configurations
- Errors in predicting chemical properties
- Difficulty in understanding atomic structure and bonding
- Inaccurate results in chemical calculations
FAQ
What is the correct format for a subshell notation?
A correct subshell notation consists of the principal quantum number (n), the azimuthal quantum number (l), and the magnetic quantum number (ml). For example, 1s, 2p, and 3d are all valid subshell notations.
What are some common errors in subshell notations?
Common errors include using incorrect letters (e.g., “f” instead of “d”), using incorrect numbers (e.g., “0s” instead of “1s”), and omitting the principal quantum number.
What are the consequences of using incorrect subshell notations?
Incorrect subshell notations can lead to misinterpretations of electron configurations and errors in predicting chemical properties.